Neurothreads
Repairing damaged brains
A researcher in Geneva wants to repair brain damage using threads laden with nerve cells. He has already developed the material he needs, but the technology faces big obstacles.
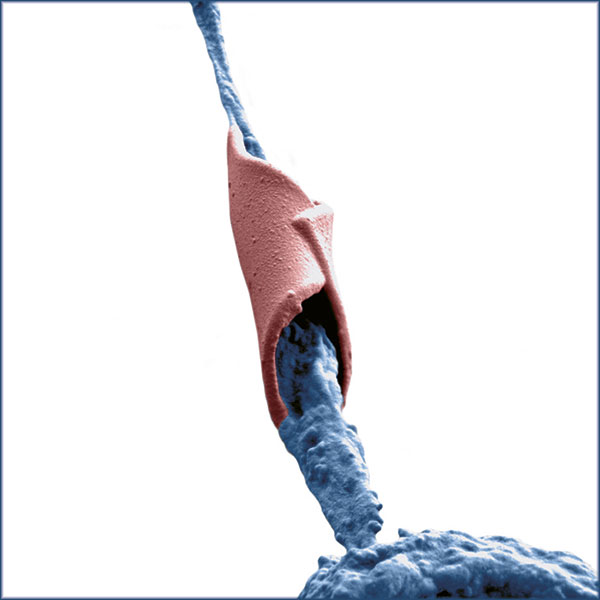
The gel (blue) protects the nerve cells (brown) when they are implanted. This is a coloured image made with an electron microscope. | Image: Aleksandra Filippova
Brain cells that die when someone has a stroke are gone for good. So are the cells that are destroyed by Parkinson’s and other neurodegenerative diseases. The gaps that then arise in the brain cannot simply be stitched up like holes in a sock.
But stitching up these gaps is precisely the approach being taken by Thomas Braschler, a materials scientist at the University of Geneva. He has developed an innovative gel that he wants to inject in the form of thin threads into the damaged areas of the brain. He first covers the material in nerve cells that he hopes will replace the dead tissue, create new connections, and produce neurotransmitters.
For three years, Braschler and his team have been working on finding the most suitable carrier material in order to be able to transport replacement cells into the brain, undamaged and with extreme precision: “It’s not so easy to implant nerve cells, because they have long extensions that are easily torn off”, says Braschler. “But we’ve largely solved this problem now”.
Successful injections into mouse brains
The neurothreads he has developed are roughly half a centimetre long and about as thick as sewing thread. They are made of a sponge-like gel, based on cellulose and produced at temperatures below zero. They are hollow inside, and create a stable structure for nerve cells to settle on. At the same time, the material is elastic and soft so it can be compressed easily and injected through a syringe. The results of the initial experiments are encouraging: when embedded in this gel structure, the fragile nerve cells survived being injected into the brains of mice, and also remained alive for at least a month afterwards.
The idea of developing a carrier material like this is nothing new. “Regrettably, up to now, hopes that such implants might be able to reconstruct neural tissue have been fulfilled in only the rarest of cases”, says Martin E. Schwab, a professor of brain research at the Institute for Regenerative Medicine at the University of Zurich. He himself has experimented with all kinds of materials to try and bridge spinal cord injuries and to repair the damage left by strokes. “The central nervous system is very unwilling to integrate implants, and does it very badly”. Such efforts have often resulted in inflammation processes that either broke down the material or closed it off.
Braschler admits that such adverse reactions will have to be clarified in large-scale studies. But in his small-scale series of tests, he has not noticed any significant closing-off of the neurothreads. And in fact, it is his stated aim that this material should be broken down – though not before the new nerve cells have integrated themselves in the surrounding tissue.
But Schwab is sceptical for other reasons too: “The idea of trying to reconstruct a piece of the cerebral cortex is a very bold one”. This is because the implanted nerve cells don’t just have to survive, but also have to construct meaningful connections with the neural cells in their vicinity. “If a piece of the cerebral cortex is missing that steers, say, an arm or your powers of speech, it’s not enough just to inject a few million cells, because they don’t know what they’re supposed to do”. And it’s true that Braschler has not yet proven that the nerve cells he injects can truly build up connections with neighbouring neural cells. But the initial experiments to investigate this are already up and running.
The danger of tumours
Braschler is also pioneering in his choice of the nerve cells he transplants. He takes embryonic stem cells and subjects them to a special treatment inside the neurothreads in order to turn them into mature nerve cells that can produce the neurotransmitter dopamine. Braschler believes that implanting these cells could help Parkinson’s patients in particular, because their lack of dopamine in the brain is what leads to the motor disorders and other symptoms typical of their disease.
Hans Rudolf Widmer is a professor of neurosciences and runs the research lab at the university clinic for neurosurgery at the Inselspital in Bern. In principle, he finds Braschler’s idea interesting. He has been looking at ways of treating Parkinson’s with neural cell implants for more than 25 years now. Although this approach is promising, any prospect of using it on patients is still far off, and it is fraught with difficulty. Many researchers use embryonic stem cells for these transplants, but they then risk these cells proliferating in the brain in an uncontrolled manner, forming tumours.
“Braschler gets around this by using adult nerve cells. But this creates another big problem, namely that these are then rejected by the immune system”, says Widmer. He notes that Braschler’s experiments so far have been performed on mice with suppressed immune systems in which no rejection can take place.
But Braschler already has a potential solution for this problem, too. Initial experimental tests have shown that endogenous cells are not rejected in his system. At present, many research groups are working on such cells. Some are trying to create dopamine-producing neural cells from endogenous cells from patients, not embryonic stem cells. This means there will be no immune response later.
However, there is another reason why Widmer finds Braschler’s results very provisional at the moment: he has not yet proven that his nerve cells can create extensions in the brain. What’s more, he has not yet tested his neurothreads in special mice that simulate Parkinson’s. But despite all his scepticism, Widmer thinks it’s a good thing to look for innovative therapies for Parkinson’s. “It affects a lot of people and there is no cure. The only therapies for it often lead to severe side-effects over the years”.
Braschler is very much aware that many people find his ideas utopian. “But it’s important to aspire to something. And bypaths can emerge that can be pursued too if they are promising”. For example, a biotech start-up has been created with the aim of using his gel for other medical purposes – such as replacing the fatty and connective tissue that is lost when tumours are removed. “It’s like with the North Star. Even if you try and reach it, you never will. But it still helps us to keep going in the right direction”.

Thomas Braschler is a materials scientist. He knows many believe his ideas to be utopian. | Image: Daniel Rihs
Looking back, it seems that his whole career has been geared towards his current project. When he was just a school student, he ran his own chemical lab in his parents’ house and won gold and silver medals at science competitions. After studying biology, he did his doctorate in microtechnology and later worked on developing vaccines against cancer. Now he has slipped into the role of an engineer and a materials scientist.