HOW IT WORKS
Faster Li-ion batteries
Long charging times, over-heating and the risk of explosion all limit the use of rechargeable batteries. A spin-off company of ETH Zurich is able to improve things by making just a microscopic change.
Long charging times, over-heating and the risk of explosion all limit the use of rechargeable batteries. A spin-off company of ETH Zurich is able to improve things by making just a microscopic change.
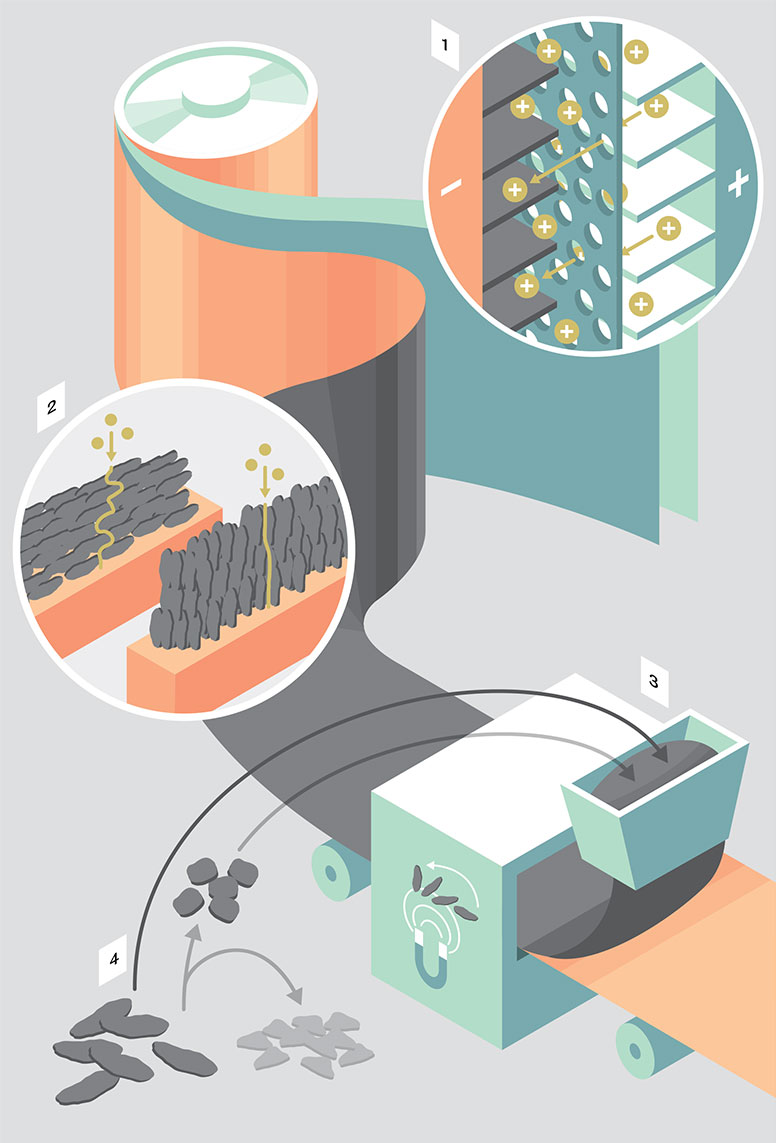
Illustration: ikonaut
4 — Outlook: saving graphite
In the natural world, graphite occurs in small flakes. These flakes have to be rounded off in the production of conventional batteries, resulting in two thirds of the material being lost. But this new, parallel alignment of the flakes means that we might be able to dispense with this wasteful intermediate step in future. Batteries would then become cheaper and more environmentally friendly.
3 — Manufacture: magnetic alignment
The spin-off company aligns the graphite flakes by means of magnetic fields. They achieve this with the usual process for coating rolls of copper foil that is a common practice in the battery industry.
2 — The solution: shorter paths for ions
The ETH spin-off Battrion has developed a technology to help charge batteries faster. By shortening the path of the ions through the graphite layer, they accumulate less and can penetrate deeper. In conventional lithium-ion batteries, the graphite flakes are oriented perpendicularly, requiring the ions to execute a zigzag motion. But the new technology aligns the flakes parallel to the flow of ions, thereby shortening the individual paths. It also means that the batteries do not heat up as much.
1 — The problem: slow charging times
In the batteries of smartphones and electric cars, positively charged lithium ions flow from one electrode to the other through a porous separator. Aluminium coated with lithium metal oxide is used on the positive pole, while copper coated with graphite is on the negative one. While the battery is charging, the positively charged ions penetrate the graphite layer. For our purposes, the faster they can do this, the better. All the same, the charging process has to be kept slow, otherwise lithium deposits will form, causing the battery to lose capacity or even explode.
