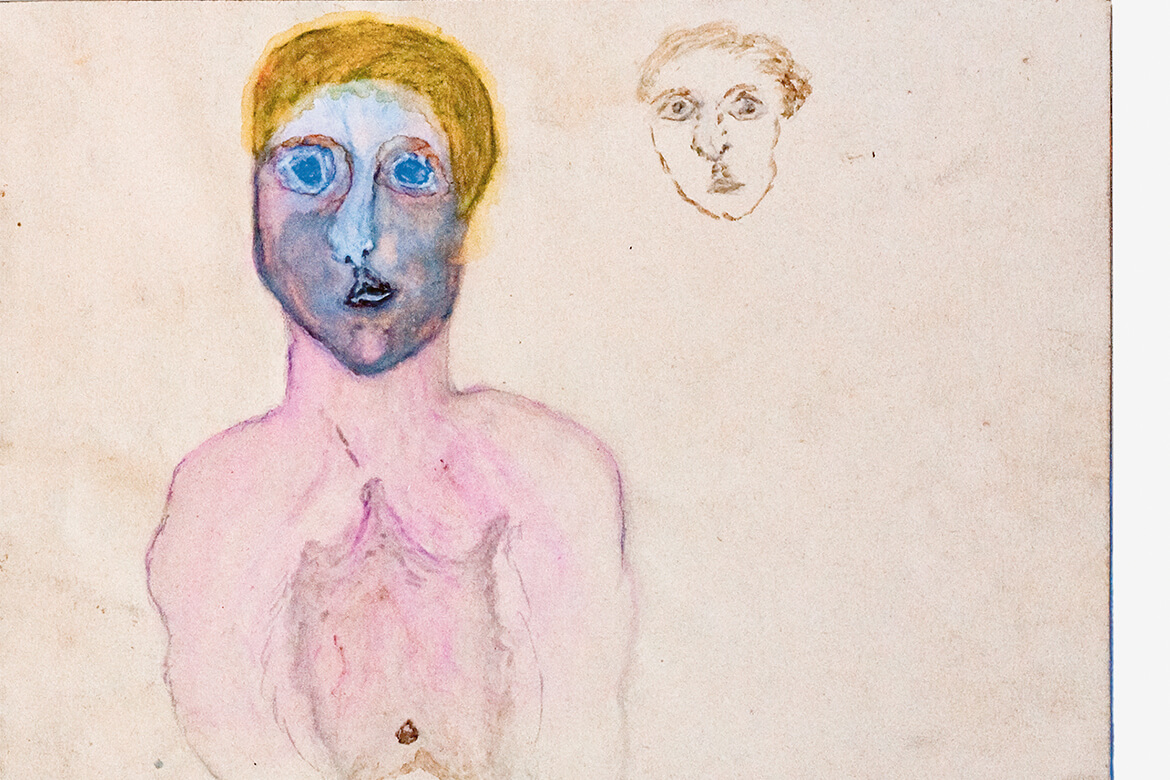Feature: Patient data
How personalised medicine saves lives – and could save even more
Genetic differences can explain why therapies help some people but not others. This knowledge is now being applied successfully in some cancer sufferers. But in future, this kind of personalised therapy could bring far greater improvements.
The data researcher Granville Metheson has shared his own, highly personal data with everyone. We can here see his heartbeat during his defence of his doctoral thesis; it varied from 40 to 120 beats per minute. | Graphic: Granville Matheson, adapted by: Oculus Illustration
When this patient – let’s call him Michael Keller – was admitted to the emergency department of the Zurich University Hospital, he could hardly breathe because of the fluid that had accumulated in his lungs. The doctors performed thoracentesis – inserting a needle through his chest wall to relieve the pressure on the lung. When they analysed the fluid, they discovered malignant cancer cells. A computer tomograph also revealed shadows in the lungs that were probably metastases. At that point, Michael Keller and his doctors knew two things (his name may be fictional, but he and his illness are very real indeed). The first was that he had cancer, and second, that his situation was acute, because the cancer had already spread, a diagnosis akin to a death sentence 20 years ago.
Even back then, doctors would have been able to categorise tumours under the microscope. “That provided them with information about the organ of origin, the type and aggressiveness of the tumour, and initial indications as to possible therapies”, says Andreas Wicki, the head oncologist at the Zurich University Hospital. But treatments were rarely successful with tumours that had already metastasised. If we include all types of cancer, just under five percent of patients survived in the year 2000 if their cancer was already at an advanced stage. Today, 20 percent of people with metastases survive. And that’s thanks to personalised therapies.
Genetic data bring hope
Today, our genes can often reveal the cause of a tumour and how best to treat it. “Cancer usually develops in response to our genetic material changing over the course of our life”, says Wicki. Today, specialists in Swiss hospitals can use modern DNA sequencing methods to render these changes visible. In order to do this, they analyse the genetic material of the tumour cells. Depending on the type of cancer, they look at between 50 and 400 genes that are known to contain carcinogenic mutations.
Today these genetic analyses are standard when the diagnosis is skin cancer, breast cancer or lung cancer. They can be used, for example, to distinguish between some ten subgroups of tumour in lung cancer, and can enable a personalised treatment of them. A more specific example would be people whose tumour growth is driven by a mutation of a protein called the ‘epidermal growth factor receptor’, who receive a drug that inhibits precisely this protein. Likewise, cancer sufferers with a mutation of the receptor tyrosine kinase ‘RET’ are given a RET inhibitor.
For patients whose tumours do not show any of the treatable driver mutations, oncologists employ immunotherapy either on its own or in combination with chemotherapy. Such targeted therapies are possible today because researchers began studying the influence of genetic differences in the early 2000s, soon after the first human genome was decoded. This enabled them in turn to start developing active substances with specific targets.
Our patient Keller also received the result of his genetic analysis one week after being admitted. It revealed that he suffered from skin cancer and that his form of cancer was best treated with immunotherapy. Since then, he has been receiving an infusion of a so-called ‘checkpoint inhibitor’ every three weeks. These drugs inhibit regulatory proteins in the immune system and prompt it to start fighting the tumour cells.
It still doesn’t work for many illnesses
Most diseases and therapies differ from person to person. We don’t all suffer equally from the side effects of medicines, we all have different levels of risk for cardiovascular and many other diseases, and, when we’re infected with something, we suffer to different degrees from the symptoms. Personalised medicine aims to leverage these individual characteristics to improve the treatment of each and every patient. So far, personalised therapies have only begun to be used in patients with certain types of cancer or with specific rare diseases, e.g., cystic fibrosis. But these therapies have huge potential and could help many more patients.
The most recent example is Covid-19. “By no means all severe cases of the disease could be explained away by the age or previous illnesses of those affected”, says Jacques Fellay, the director of the Precision Medicine Unit at Lausanne University Hospital (CHUV). Together with an international team, he has been investigating why many young people had to be ventilated or even died after being infected, despite having been perfectly healthy beforehand. To this end, he and his colleagues have analysed the antibodies and genetic information of around 1,500 patients in ICUs on five continents. They have revealed certain mutations that switch off proteins in the body’s immune response, raising patients’ susceptibility to infection. “This enables us to explain some 20 percent of atypical, severe cases”, says Fellay. They are also investigating why an HIV infection remains under control in a small number of patients, even without their taking any medication, and why people react differently to the hepatitis B virus.
Usable data are rare
But if researchers are going to apply findings like this from basic research to develop therapies for patients in the future, they have to do more than just recognise biological and genetic variations. They also have to understand the influence that these variations exert on a disease. “In order to make progress in this field, we’re primarily going to need much more usable data than we’ve had before”, says Fellay. But there are hurdles to this in Switzerland.
This is also the opinion of Christiane Pauli-Magnus, a co-head of clinical research at the University of Basel and the local University Hospital. As a consultant physician in rheumatology, she is confronted daily with the limits of contemporary medicine. In her field, there are only a few therapies available, and they are all similar in that they work for some patients, but not for others. “We don’t know why”, she says. So they have no choice but to try one therapy after another. “If we were instead able to understand the causes, we could help patients more quickly without burdening them with unnecessary therapies”.
According to Pauli-Magnus and Fellay, the above-mentioned hurdles lie both in data collection and data use. Regarding the former, essentially, patient data come from two sources, i.e., clinical studies and routine hospital data, which are generated while treating patients. These data include vital signs, laboratory values, the results of imaging technology – in short, a patient’s entire medical history. The information is stored locally in hospitals, where it is protected. “This is valuable data that we definitely ought to use”, says Pauli-Magnus. And when patients are asked if they are willing to release their medical data for purposes of research, there is generally a high degree of willingness: eight out of ten patients provide consent. The problem, however, is that only a fraction of them are ever asked about this.
Positively mediaeval
This is largely because patients in Switzerland still have to give their consent by physically signing a piece of paper, says Pauli-Magnus. This complicates the process, especially when the patients have already left for home. “If the forms are sent by post to people at home, they often end up in the bin”, says Pauli-Magnus. “In a digital world where we send private pictures and data unencrypted to the other side of the world without giving it a second thought, this legal requirement of having a handwritten document is positively mediaeval”. She would rather see the introduction of so-called ‘e‑consent’, which would allow those being treated to provide their consent electronically.
The second hurdle is the lack of standardisation in routine data. This is even a problem with seemingly simple matters, e.g., designating the female and male genders. Various codes are used for this – man/woman, m/f, male/female, and so on. This makes it difficult to compare data from different hospitals.
The Swiss Personalised Health Network (SPHN), a federally funded initiative, has been working on solutions to this problem for the past five years. The experts at SPHN have built up a network comprising university hospitals, universities, the SNSF, the Swiss Clinical Trial Organisation and other protagonists in the Swiss healthcare system. “The idea is to invite everyone to the table to construct a secure research infrastructure for exchanging data, and to develop standards for processing them”, says Urs Frey, the president of the SPHN Steering Board. This process of standardisation is currently progressing step by step, through funded projects. For example, university hospitals now accommodate data warehouses in which patient data are catalogued in a standardised manner that is regulated both ethically and legally. These warehouses are connected to each other and to the universities through BioMedIT, a recently established, secure network platform.
There are still gaps
However, the SPHN has an expiry date. The initiative will be stopped in 2024. It remains to be seen how the data infrastructure will be maintained and financed after that. Another gap is likely to arise in the near future, because the funding for longitudinal studies is also coming to an end. The SNSF funded ten similar studies up to the year 2020. These included monitoring Swiss lung or heart patients over several decades, for example. “Such data are extremely valuable because they don’t just offer a snapshot. They reveal how the health of the study’s participants develops”, says Christoph Meier, the Head of Projects in Life Sciences at the SNSF. However, the last of these studies will end in 2024 and it is still unclear how it might be funded after that. This is currently being examined internally at the SNSF.
Meanwhile, the research community would like to see a greater commitment from those responsible for the country’s health policy. “If we are going to achieve rapid advances in personalised medicine, the Swiss healthcare system will have to focus more intensively on research than it has in the past”, says Fellay. According to Wicki, a different incentive system has to be set up: “We would need the kind of funding that rewards healthcare providers who generate research data of high quality that can be shared and exchanged”.
At least Keller has a significantly better chance of survival thanks to his personalised therapies. Before they were developed, less than ten percent of patients with metastatic melanoma were alive ten years after diagnosis. Today, that number has risen to more than 50 percent.